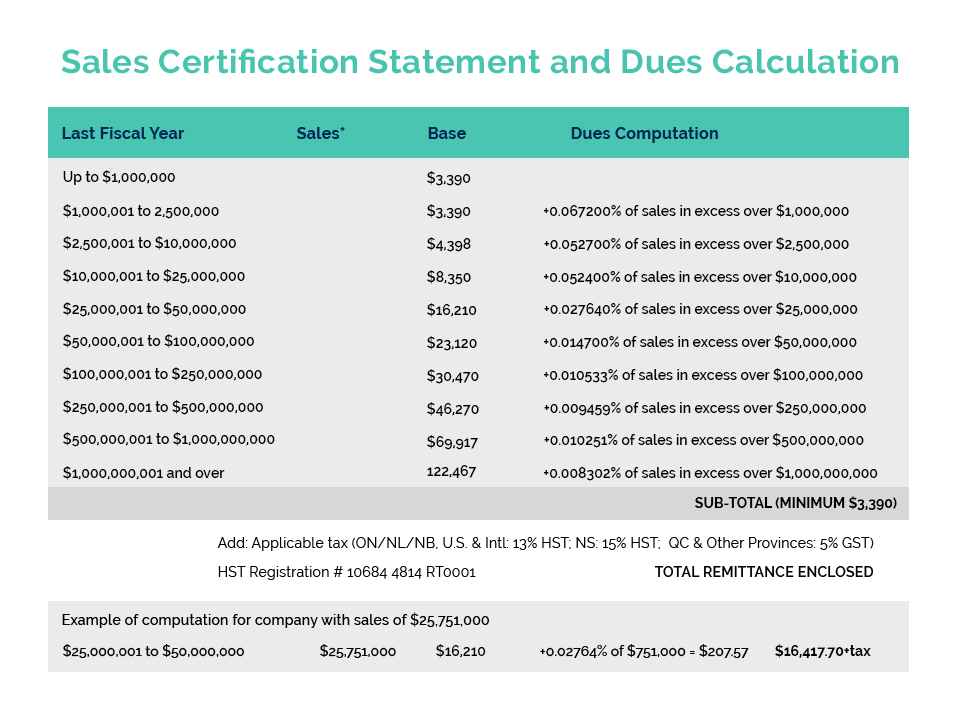
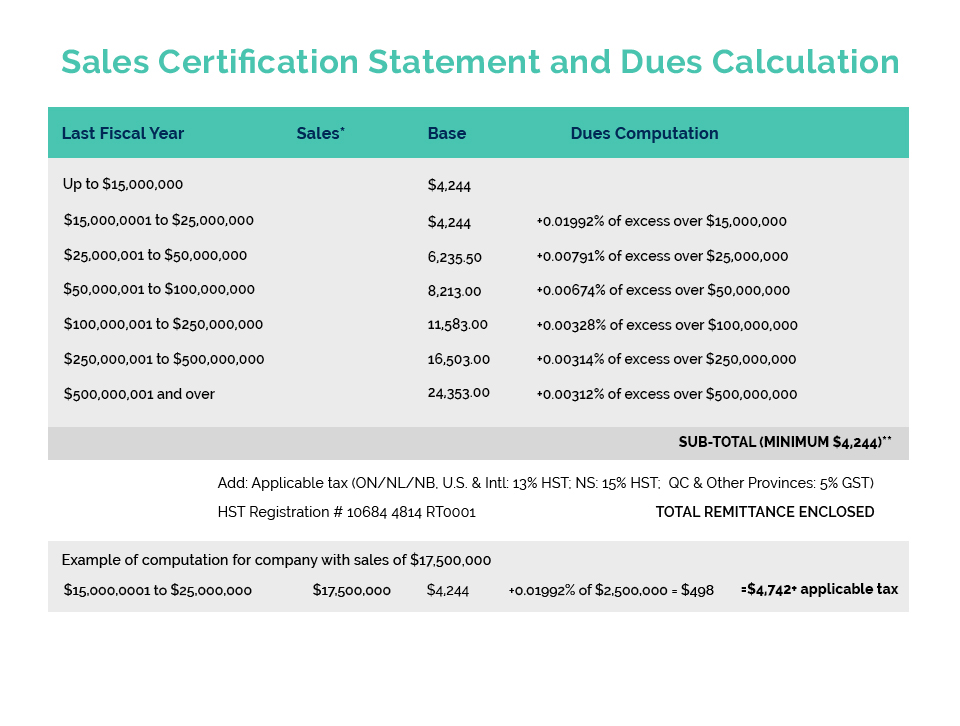
CA Canada continues to pursue the establishment of a new Government issued or authorized GMP compliance certificate to facilitate the export of Canadian manufactured cosmetic products to China. This compliance certificate is required by China to exempt imported cosmetic products from its animal testing requirements and as such provides a significant opportunity for the Canadian-based cosmetic manufacturing industry.
The provision to enable this animal testing waiver was officially implemented on May 1, 2021. Although we have had many meetings with a multitude of Health Canada staff over the past few months to prepare for May 1, the issue has yet to be resolved, which is creating a growing frustration among our cosmetic manufacturing members who see this as an opportunity to increase their manufacturing and exports.
In our discussions with officials, it has become evident that a roadblock in implementing the required “compliance certificates” lies within the current administrative structures of Health Canada and which branch has responsibility for what.
Specifically, Health Canada DOES NOT currently require the licensing of manufacturing facilities producing cosmetics. However, Health Canada DOES issue Drug Establishment Licences (DEL’s) for the manufacturing of non-prescription drugs, as well as Site Licences (SL’s) for natural health products. As most Canadian manufacturing sites for cosmetics already have a DEL or SL, as they also make drugs or NHPs, using these existing licenses should provide more than a sufficient basis for issuing a “compliance certificate” for cosmetics manufactured in these sites.
Consequently, one branch of Health Canada will have to rely on the work of another branch to issue such a “compliance certificate” for these cosmetics, and the decision to authorize this initiative must come from a high level that crosses the two branches.
To this end, CA has sent a further letter to the Ministers of Health and International Trade requesting this matter be given the necessary attention at a senior enough level between the two branches to advance this initiative and implement our suggested solution as soon as possible. CA will be meeting with Health Canada officials again this Friday and will continue to keep members apprised of developments.
We would also urge all CA member companies with an interest in these certificates to send a letter to the two Ministers supporting this effort and stressing the urgency to your company. A draft letter and e-mail addresses for the two Ministers is available HERE.