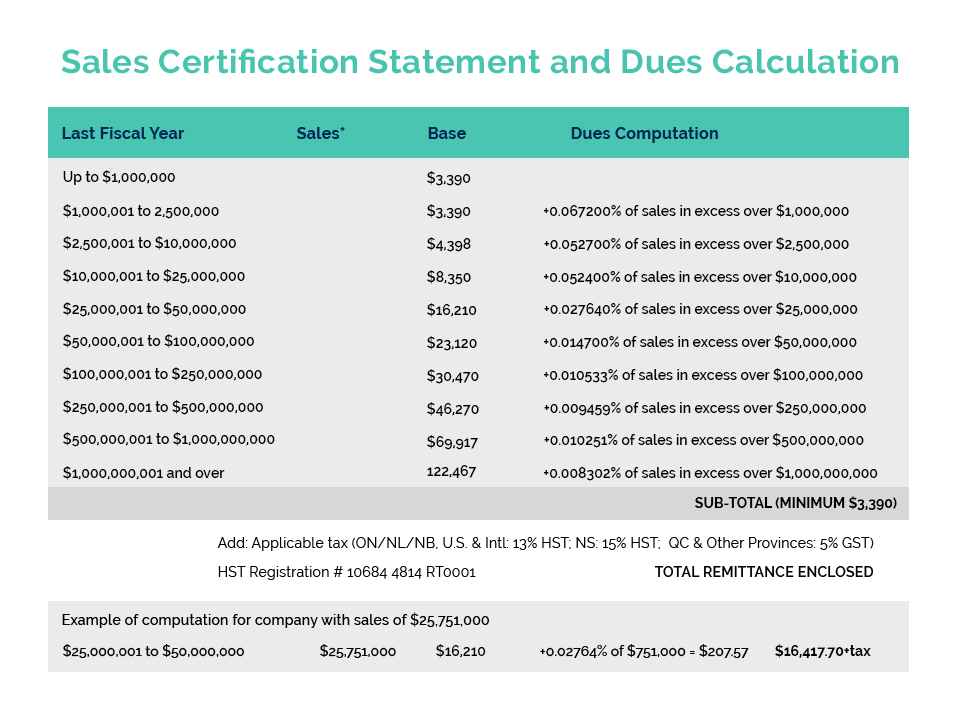
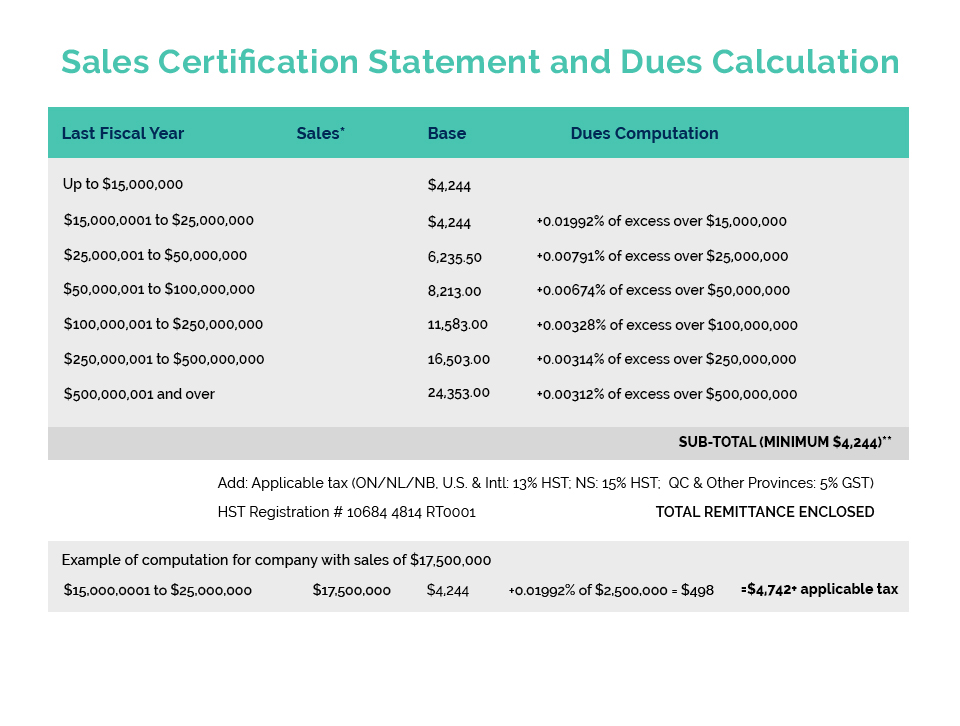
WHAT YOU NEED TO KNOW:
Quebec and Ontario Agree to Issue Interim GMP Certificates For Cosmetic Exports to China
As you may know China recently introduced an exemption to their regulatory requirements for animal testing on imported cosmetic products.
To qualify for this exemption exporters need to provide a government issued certificate of GMP compliance. Certificates must be provided for sites in which cosmetic products are manufactured and must be specifically issued by a government authority in the countries from which the product is manufactured.
The challenge with this requirement is that that there are very few jurisdictions around the world (if any) where such certificates are issued by government. CA has been working with Health Canada as the regulatory authority to establish a process to support the issuance of such a certificate.
Although we expect Health Canada to shortly approve such a certificate, trade/economic ministries in both Quebec and Ontario have agreed on an interim basis to issue a certificate with respect to GMP compliance for cosmetic exports to China.
These certificates are intended for facilities in their respective jurisdictions that:
- Manufacture cosmetic products and have an ISO Certification (from auditors/certifiers accredited by Standards Council of Canada); and/or
- Manufacture cosmetic-like Natural Health Products and hold a Site License (SL); and/or
- Manufacture cosmetic-like drug products and hold a Drug Establishment License (DEL).
CA can help you prepare the necessary documentation and submit an application to the appropriate government office in each province. Please contact CA for further information.
FURTHER CONSIDERATIONS YOU NEED TO KNOW:
- Quebec-issued certificates have been accepted by Chinese authorities (with a few shipments leveraging this certificate being cleared for import into China without animal testing)
- There is not yet a precedent for the certificate from Ontario being accepted; however, this certificate leverages the approach used in the Quebec-issued certificate
- All of these provincial-issued certificates are intended as an interim option for consideration as we finalize a more appropriate and thorough solution with Health Canada
- Overall, despite the success with a Quebec issued certificate, none of these certificates are guaranteed, as Chinese authorities have yet to make clear what specific information and supporting evidence they will accept on a consistent and long-term basis.
WHAT YOU NEED TO DO:
If your site holds a valid ISO Certificate, SL and/or DEL, contact your CA Canada Certificates Team to explore these opportunities in further detail.
Should your company manufacture cosmetics for export to China in another Canadian province or territory, please advise CA and we will pursue a similar interim certificate in that jurisdiction.
Contact Info:
Deborah Kwinter, Manager, Export Certificates Program
Email: dkwinter@cosmeticsalliance.ca
Phone: 905 580 1400