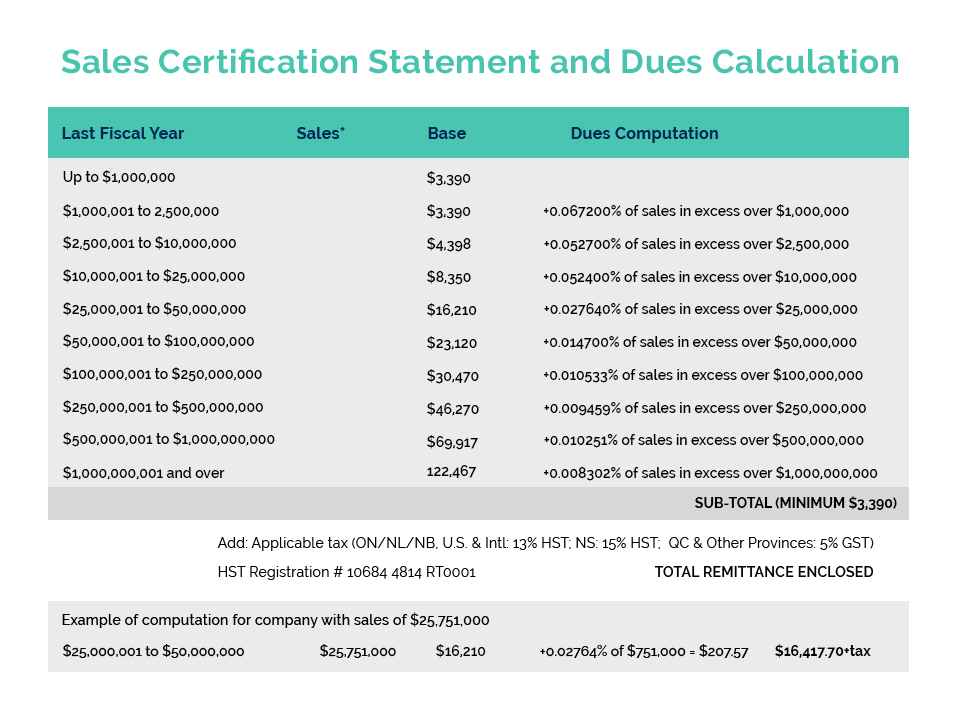
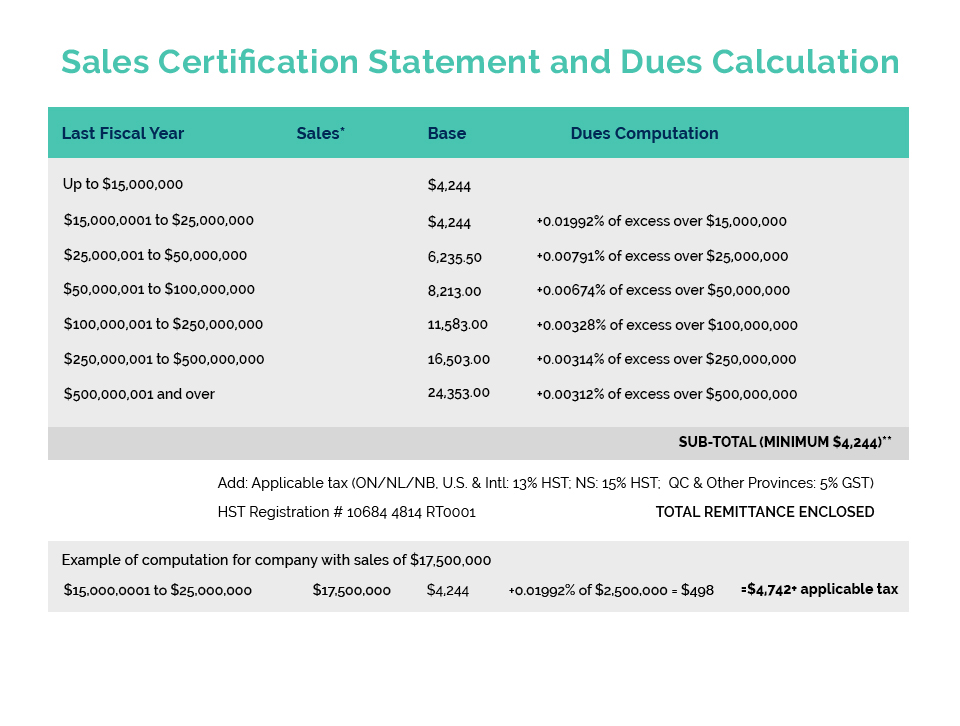
The 17th annual meeting of the International Cooperation on Cosmetics Regulation (ICCR) was held July 11 – 13 in Brazil. Dr. Alex Campos (ANVISA – Brazilian Regulator) and Mr. Joao Carlos Basilio (ABIHPEC – Brazilian Cosmetic Trade Association) welcomed the world to the first in-person ICCR meeting since 2019, when Canada last hosted this important global forum for joint regulator/industry dialogue in Montreal.
ICCR brings together regulators and industry representing Brazil, Canada, Chinese Taipei (Taiwan), Europe, Israel, Japan, South Korea and the United States (Full Members). In addition, representatives from Capo Verde, Egypt, China, Saudia Arabia, and the United Kingdom joined the dialogue as Observers. Collectively, these jurisdictions represent in excess of 65% of the global cosmetics market.
In launching this year’s Joint Dialogue, both ANVISA and ABIHPEC reflected on the importance of the forum to share experiences, best practices, and technical/scientific knowledge to support regulatory convergence around the world. In welcoming the world, Dr. Campos and Mr. Brasilio also recognized the essentiality of cosmetic and personal care products to support health and hygiene, promote self-esteem, and enable a higher quality of life.
ICCR was founded some 17 years ago by its original four members – Canada, the European Union, Japan, and the United States – for the purpose of coordinating the regulation of cosmetics to enhance consumer safety and align regulations to facilitate trade. Industry, through our trade associations in the ICCR jurisdictions, participate with regulators in joint working groups throughout the year to discuss specific issues of mutual concern and make recommendations that can be endorsed and published by the group of ICCR regulators. These papers – such as allowable levels for impurities of certain substances such as lead (i.e. 10 ppm) – often then become a standard used or referenced by other regulators and industry around the globe.
At this years meeting, topics for discussion included improving consumer communications and integrated strategies for safety assessments. Proposed new topics for work during the coming year include possible survey work on PFAS, as an emerging ingredient/contaminant of concern. In the industry-only meetings, excellent dialogue regarding the potential to add digital labelling to the ICCR agenda was also discussed, where it was confirmed that this is an important industry need, moving forward.
In addition to meeting with industry stakeholders, ICCR regulators also hold an open forum with any interested stakeholder. This year stakeholder participation was headlined by a presentation by the International Collaboration on Cosmetic Safety (ICCS) in which President & CEO Erin Hill introduced the ICCS to ICCR and highlighted opportunities for collaboration on NAMs and facilitating regulatory acceptance of NAMs. This was followed by presentations from Cruelty Free International and Humane Society International, who continue to profile and seek cosmetic animal testing bans internationally. The session closed with a presentation from the Brazilian Society of Professionals of Clinical Research.
Over the years, the ICCR meeting has expanded to include an industry symposium led by the hosting jurisdiction. The symposium takes advantage of ICCR-regulators (both full ICCR members and observers) being in the same place at the same time. This provides a unique opportunity for industry to advance or profile a topic or development that could spearhead ‘big picture’ policy dialogue on matters that might not necessarily fit within the technical realm of the ICCR deliberations, but nonetheless are critical to facilitate/support international trade and/or regulatory convergence. This year’s symposium was titled “Building Scientific Confidence in the Use of Non-Animal Methods for Safety Assessment of Cosmetics” and featured presentations from Erin Hill (ICCS) – Collaborative Efforts to Advance Next Generation Risk Assessment (NGRA) for Cosmetics; Dr. Rodrigo De Vecchi from L’Oreal – NAMs for Skin Sensitization; Dr. Matthew Dent from Unilever – Perspectives on NAMs for Systemic Toxicity; and Dr. Joao Barrosa representing the European Commission (Joint Research Centre) – Building Confidence in Non-Animal Approaches.
Importantly, ICCR remains a critical platform for enhancing international regulatory alignment and standards for our industry – a key objective in facilitating international trade and manufacturing efficiency.