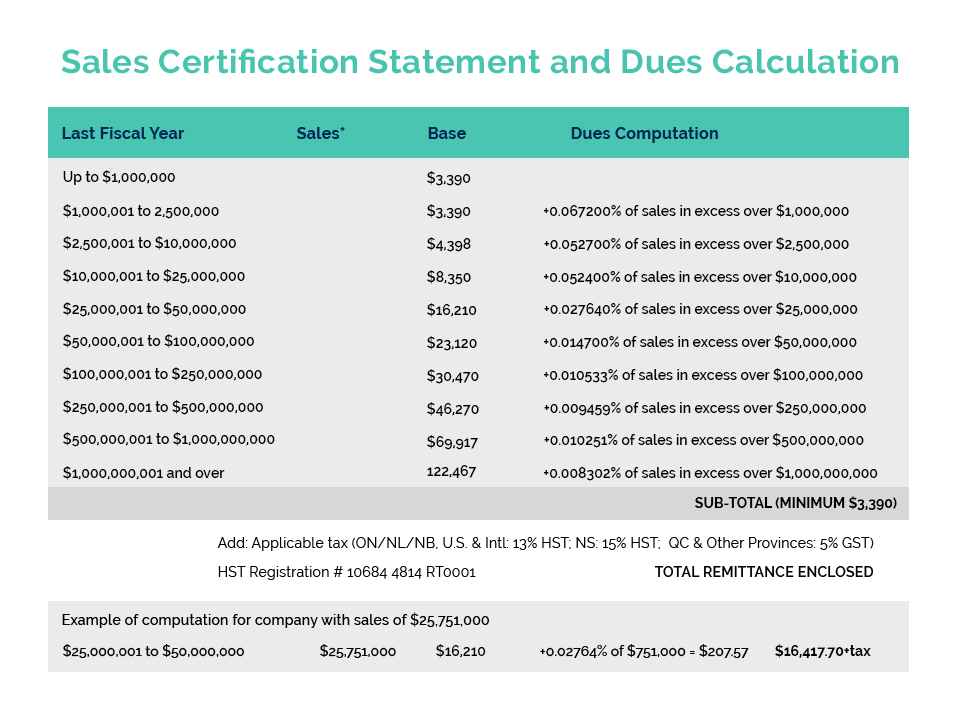
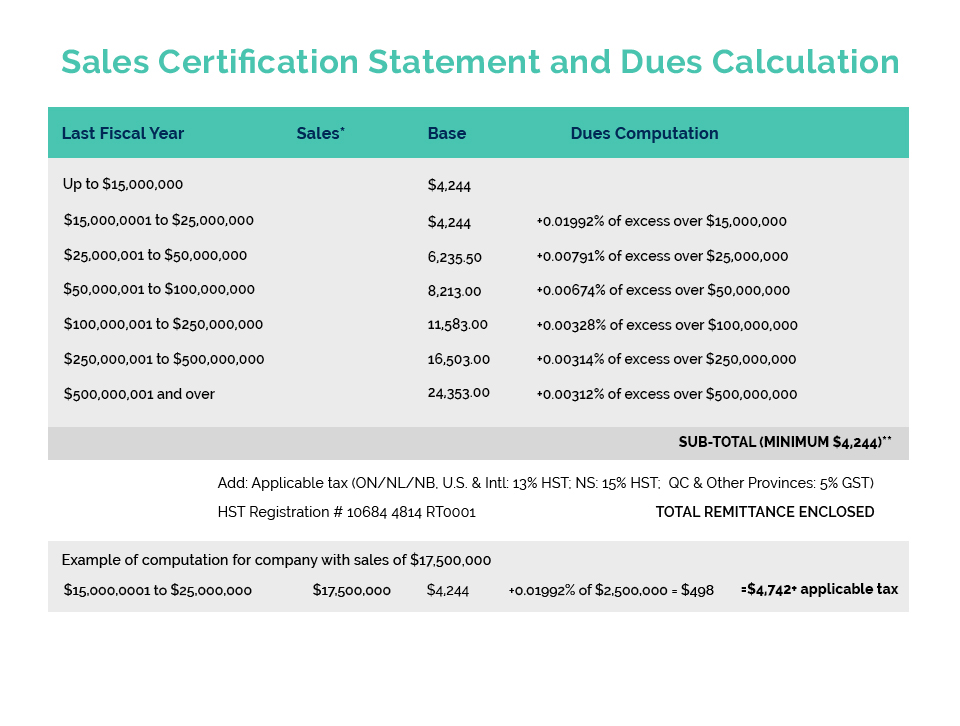
Cosmetics Alliance Canada Export Certificates are for companies which manufacture and/or sell in Canada and are looking to export globally. They are NOT for companies importing into Canada. For information on requirements for the sale of cosmetics in Canada, please visit Health Canada’s Cosmetics website.
As a service to the cosmetic industry, Cosmetics Alliance Canada offers a certificate program to facilitate international trade. This certificate program, which includes Certificates of Free Sale, Good Manufacturing Practices Certificates and NHP International Trade Certificates, is offered to both member and non-member companies. For close to 100 years, Cosmetics Alliance Canada has been the leading Canadian association for the cosmetics and personal care products industry and for over 25 years CA has been issuing Certificates destined to over 90 countries.
1. Certificates of Free Sale (CFS)
Certificates of Free Sale are Affidavits signed by a qualified Cosmetics Alliance Canada specialist attesting that, to the best of the specialist’s knowledge, the personal care products in question are identical to those sold throughout Canada. A CFS is often requested by a foreign government to ensure that Canadian imports are in compliance with applicable Canadian Provincial and Federal law.
2. Good Manufacturing Practices (GMP)
Good Manufacturing Practices are guidelines for manufacturers to follow regarding production. These are designed to control and streamline the influence of outside factors on the quality of production. GMP Certificates are affidavits attesting that the manufacturing of the products in question are adhering to these guidelines. For additional information regarding personal care product GMPs, please reference ISO 22716:2007, Food and Drug Regulations, Part C, Division 2 “Good Manufacturing Practices” and Natural Health Product Regulations, Part C “Good Manufacturing Practices”.
CA can issue GMP certificates for Canadian manufactured products sold through Amazon USA and Canada.
3. NHP International Trade Certificates (ITCs)
Companies who export Natural Health Products (NHP) from Canada are often asked by foreign customers or regulatory authorities to provide certification relating to products that are subject to Health Canada’s NHP Regulations. An International Trade Certificate (ITC) is a document prepared by Cosmetics Alliance Canada that contains information pertaining to a product’s or site’s regulatory and marketing status in Canada and confirms that the product or site has met the regulatory requirements of the Natural Health Product Directorate of Canada (NHPD) . There are two types of ITCs: one for Natural Health Products and the other for Good Manufacturing Practices Compliance. Cosmetics Alliance Canada will issue ITCs within a period of 10 business days at a cost of $199 each Certificate. Volume discounts may apply. For all ITC requests and more information, please contact Deborah Kwinter at dkwinter@cosmeticsalliance.ca or 905-580-1400.
Please note, Cosmetics Alliance Canada provides ITCs for “cosmetic-like” NHPs which include acne therapy, anti-dandruff products, fluoride-containing anti-caries products, medicated skin products, diaper rash products, antiseptic skin cleansers, sunburn protectants, skin whiteners and tooth whiteners. Please contact us for further information or clarification.