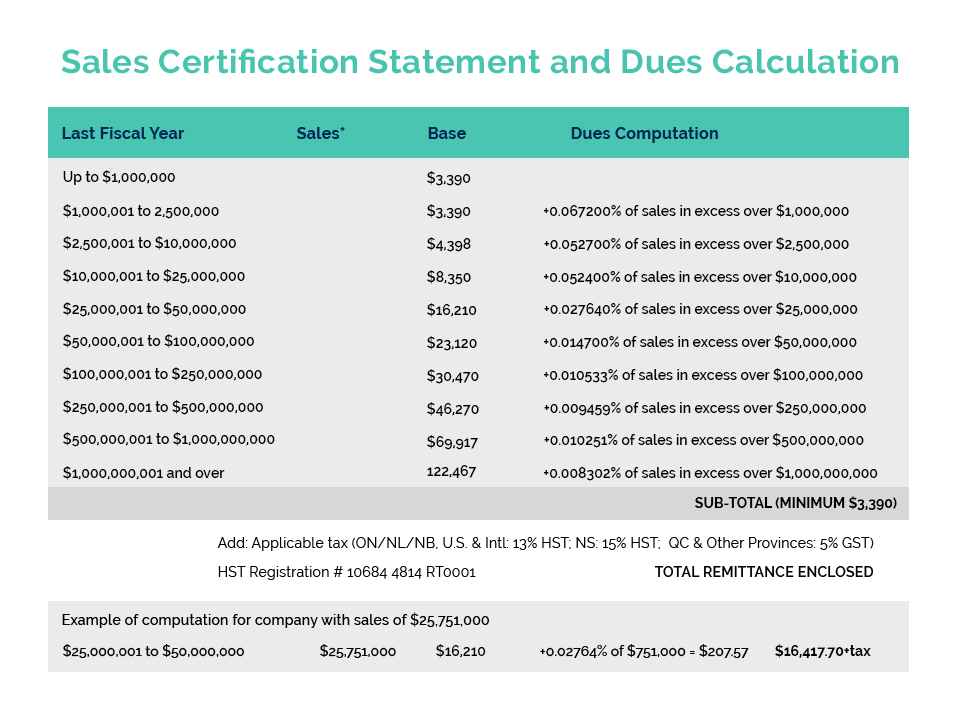
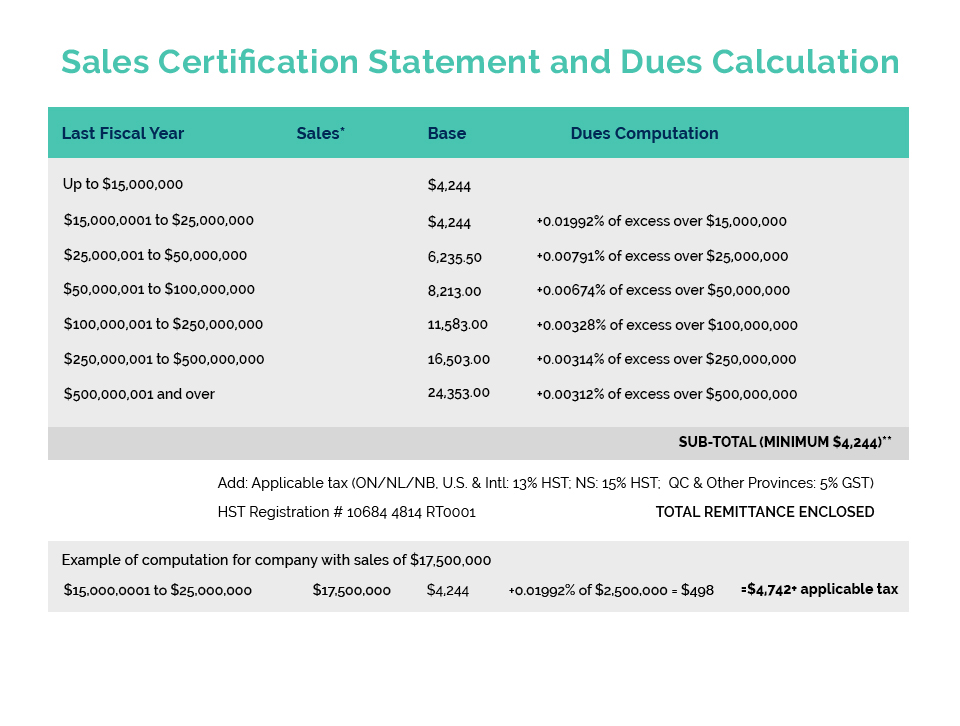
On July 16, Health Canada opened a 45-day consultation process to gather stakeholder comments and feedback regarding potential amendments to the Cosmetic Regulations. This was not a surprise as the Consumer and Hazardous Products Safety Directorate announced at CA’s Spring Virtual Regulatory Workshop, as part of their Forward Regulatory Plan for 2021-2023, plans to amend the Cosmetic Regulations to require the disclosure of specific fragrance allergens on product labelling and to enhance the regulatory oversight of cosmetics.
The Pre-Consultation Notice provides an overview of the amendments under consideration and includes a detailed Questionnaire for interested stakeholders to complete. Proposed amendments include:
- Adding a requirement to disclose specific fragrance allergens on labels of cosmetic
- Allowing flexibility for the disclosure of ingredients and specific fragrance allergens on labels of small packages
- Improving oversight of cosmetics by:
- clarifying terminology and improving the level of detail for information submitted in a cosmetic notification, in order to facilitate its use for risk management purposes;
- enhancing compliance and enforcement oversight with regard to who is responsible for responding to evidence of safety requests for cosmetics; and
- Addressing administrative updates.
The pre-consultation will be open for 45 days (ending August 30th). CA has asked Health Canada to consider an extension as the consultation period is during the summer holiday season. Although the deadline is unlikely to change, Health Canada has indicated that some latitude with be considered for individual submissions upon request.
CA has already approached Health Canada to ensure that the consultation is undertaken within the context of the Self-Care Framework and not in isolation as some of the proposed changes can also affect products that are regulated as non-prescription drugs or natural health products. With the implementation of the Framework now underway, CA will again be raising with Health Canada the need to consolidate the regulatory administration of cosmetics, OTC drugs, and NHPs into one Directorate. (The Cosmetic Regulations are currently administered by the Consumer and Hazardous Products Safety Directorate while OTC drugs and NHPs are administered by the Natural and Non-prescription Health Products Directorate.)
We encourage members to review the Pre-Consultation Notice and to respond to the accompanying Questionnaire as appropriate. CA is working with our Product Compliance & Market Access and Facility Compliance & Manufacturing Committees to consolidate a comprehensive industry response on behalf of our members.