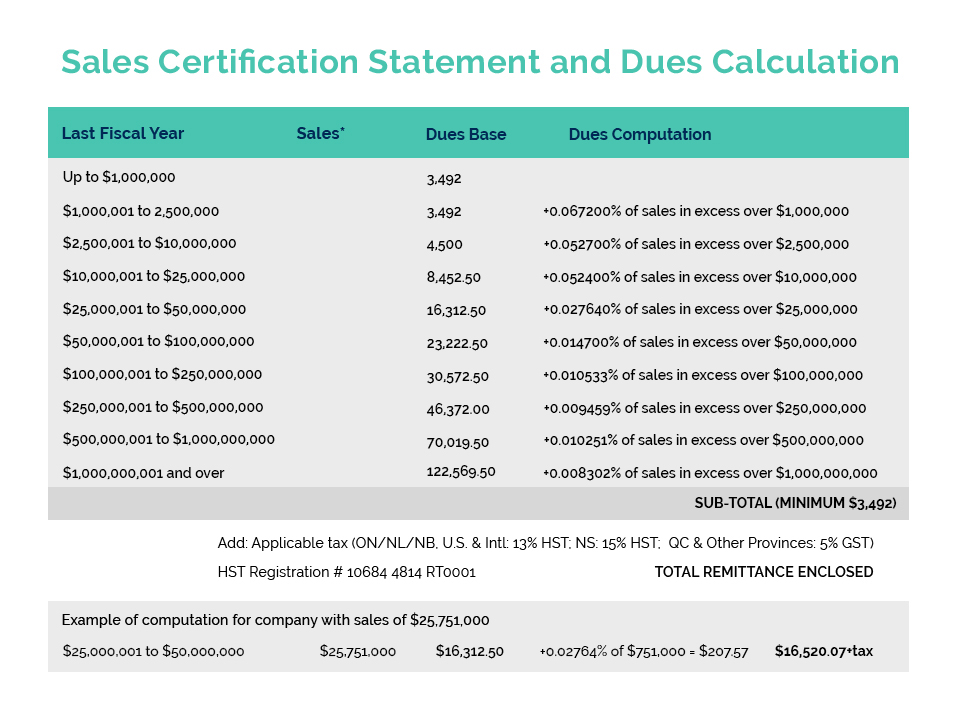
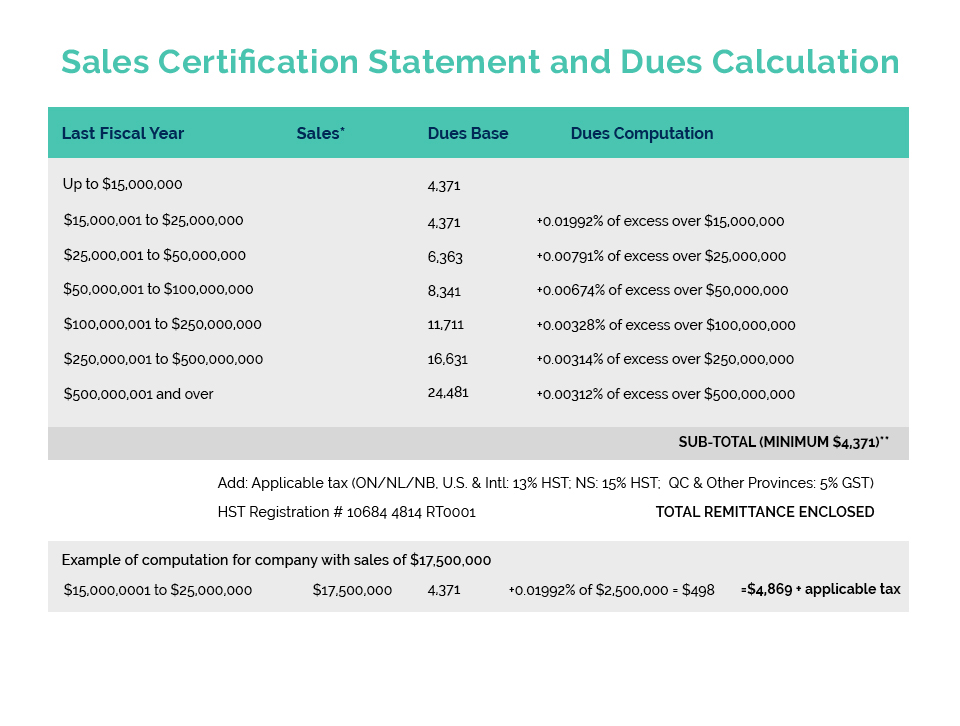
CA was in the Federal Court of Canada last week to monitor an application for judicial review by Le-Vel Brands, LLC of a “Final Decision” by Health Canada to classify the company’s “Thrive DFT” patch as a natural health product. The product first entered the market several years ago as a “cosmetic” and there are no safety or health issues which have been raised by Health Canada, only that it should have applied for approval as a “Natural Health Product” and so is non-compliant. It also appears that over a period of several years various concerns raised by NNHPD, including a link to Le-Vel’s U.S. website where differing claims were made about the product, were addressed by the company and, as Le-Vel’s council argued, the notice sent by Health Canada advising of their “final decision” was not clear as to the basis for the classification nor that the product as used and promoted in Canada fit within the definition of a natural health product.
CA President Darren Praznik, who attended the virtual hearing as an observer, noted that there are several issues that could be of concern to industry depending on the outcome. One was clearly the statutory definitions of “natural health products” and “cosmetics” and whether regulators can extend those definitions through their “interpretation” beyond the words of the statute enacted by Parliament. Another is the degree of explanation or specificity required in formal decisions issued by regulators. A comment by the Government’s council suggesting that claims made about a product in another jurisdiction – in which they may be allowed – even if the company is not making the claim in Canada and does not provide or promote any link to a foreign website, could still be considered by regulators if Canadians could find it on the internet. This, of course, would be of great concern and could be easily seen as an overreach of national regulatory authority requiring a larger policy discussion with Government.
Most surprising to Praznik was that this case is even being pursued given that Health Canada is in the process of implementing the Self-Care Framework. “Under the Framework”, noted Praznik, “whether a product was classified as a natural health product or a cosmetic would not really be of much relevance as it would be subject to the same regulatory requirements based upon which risk category it was in. In the current siloed model, however, this looks like a fight between two directorates as to who has jurisdiction.” CA expects that responsibility for “cosmetics” will eventually be merged into NNHPD so that these kind of issues will be eliminated.
The judge in the case is in the process of writing her decision. CA will update you when it is issued.