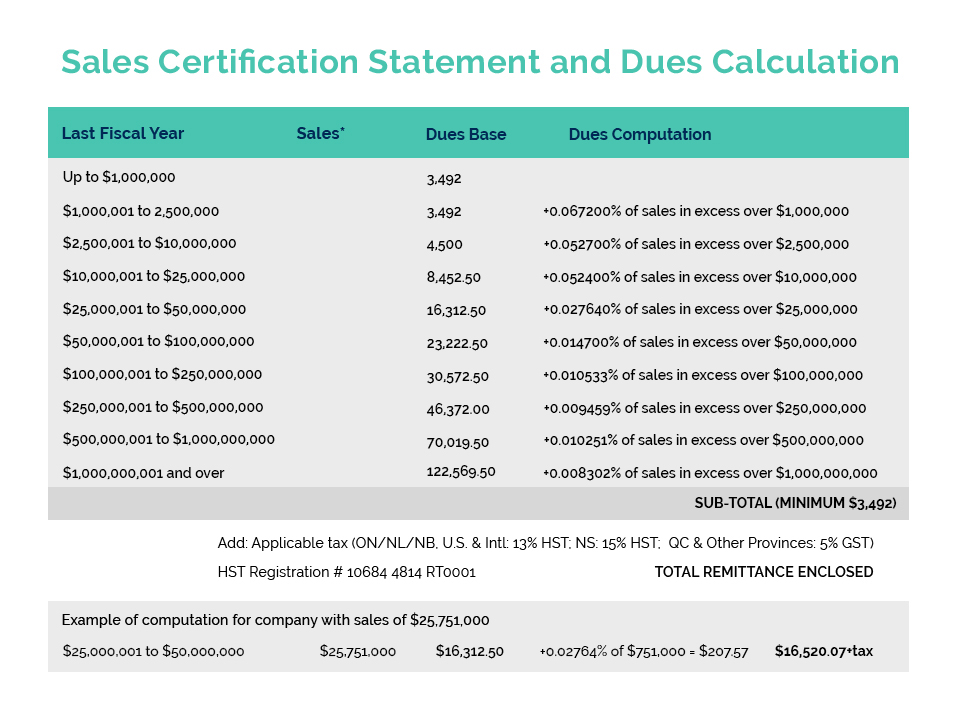
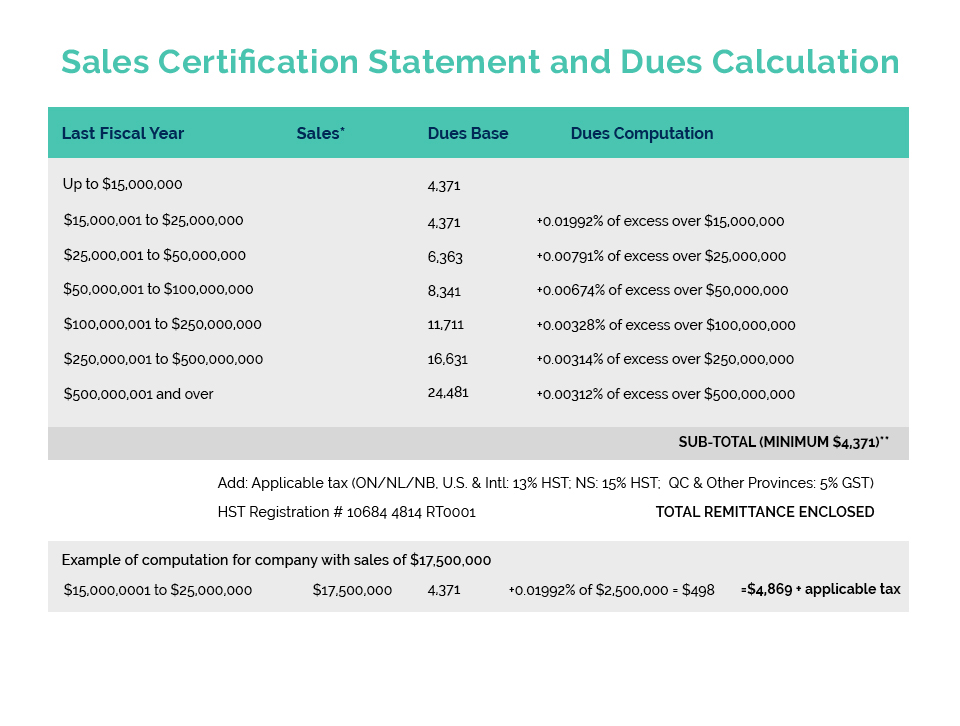
In a meeting with Dennis Price (Director General) and Geoff Barrett (Director) of the Consumer and Hazardous Products Safety Directorate (CHPSD), responsible for Health Canada’s Cosmetics Program, CA’s Darren Praznik and Beta Montemayor confirmed HC’s commitment to work with any company who will have difficulty in meeting the April 2026 ‘Coming Into Force’ Deadline for both including the initial 26 allergens on their product labels and selling through their existing stock at retail.
This has been an issue for member companies who produce and label product for a North American supply chain where this has not been a requirement until now. Companies who produce products for an international supply chain which includes the European Union would have already had to meet this requirement which has been in place in Europe since 2009. An expanded list of some 81 allergens, inclusive of the original 26 will also be required to be included on labels (including sell through) by August 2028, in both Canada and the EU.
To date, several member companies have either met with Health Canada or indicated their interest in doing so to provide a compliance plan if they will not be able to comply with the April 2026 deadline. Health Canada has indicated it may consider making an administrative policy extending the deadline-rather than dealing with individual companies – IF there are a significant number of companies who will be unable to comply by the deadline.
CA is offering to assist any member company who would like to meet with HC on this matter and has developed a general template of information required for a compliance plan. We are also prepared to accompany any member in their meeting with Health Canada. Any interested companies should contact bmontemayor@cosmeticsalliance.ca to discuss this option further.
In addition to pursuing these options for the immediate labelling challenge, HC officials agreed that it would be useful to both parties to use this experience, as well as the extensive data developed by CA and our member companies, to develop some form of “guidance” setting out the ranges of time required by companies for the various activities required to implement label changes. This could include segments for such activities as obtaining information from suppliers; making business decisions as to whether to re-formulate or discontinue the product; designing and planning a label change; making the actual label change; distributing the new product; and, of course, a reasonable sell-through period to avoid the costs (including environmental costs) of undertaking a recall of unsold product. This kind of information is, of course, essential in undertaking the cost/benefit analysis required by the federal Treasury Board for any regulatory change. The current experience with allergen labelling has clearly demonstrated a failure to take this critical information into account and is largely responsible for the current difficulties being experienced by many companies.
If your company cannot meet the 2026 deadline for labelling the 26 fragrance allergens, and you are interested in potentially developing a Compliance Plan with Health Canada, please contact Beta Montemayor at bmontemayor@cosmeticsalliance.ca for more information and assistance.