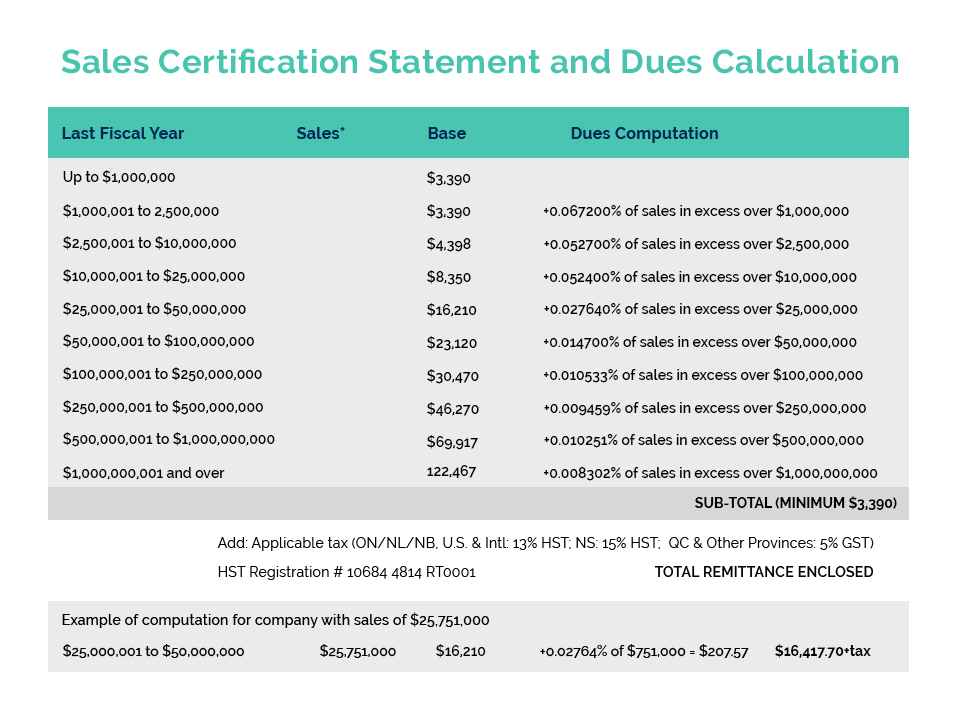
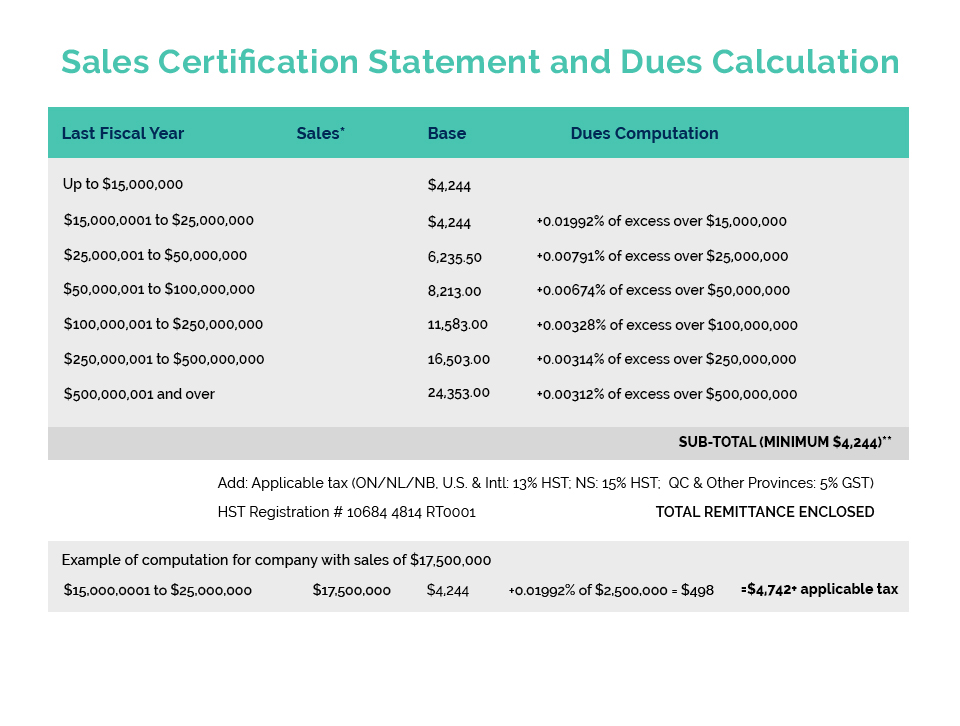
- This event has passed.
Webinar – ISO 22716 (GMP’s for Cosmetics) Audits: Top Non-Conformities and Observations
December 13, 2021 @ 12:00 pm - 1:00 pm

The webinar will focus on:
- Understanding the relation between ISO 22716 and the Canadian Cosmetic Regulations
- Learning the top non-conformities identified during SGS ISO 22716 audits
- SGS ISO 22716 GMP training opportunities
Agenda
- Introduction
- Canadian Cosmetic Regulatory Perspective
- Compliance versus Certification
- ISO 22716 Outline
- Top Non-Conformities Identified during SGS audits
- Next Steps for the ISO 22716 standard
- SGS Training Opportunities
- Q & A Session
About the Speakers

Technical Manager /Senior Auditor
SGS Knowledge Solutions
Pharmaceutical, Cosmetics, and Personal Care (PCPC)
SGS North America
Over 30 years of Quality and Project Management Experience with over 25 years of Leadership Experience in the Pharmaceutical, Cosmetic, Personal Care, Food, and Medical Device Industry
Specialties: QA, QC, Supplier Qualification and Management, CMC, Quality Management, Project Management, Certification Auditing, Vendor Auditing, Internal Auditing, Analytical Testing, QC Laboratory Management.

Mohit McLaren
Sales Manager-Canada Pharmaceutical, Cosmetics
and Personal Care Products
SGS North America
Mo has over 10 years of technical audit and sales consultancy with SGS Canada is responsible for the delivery of Supply Chain and Quality Compliance solutions to the Cosmetic and Pharma industry. Some of his certifications include:
Medical Pharmaceutical certification EuGMP, cGMP, ISO 22716, 21CFR 210/211, ICH Q7, GLP, GCP
Food Safety, BRC, HACCP, FSSC 22000 Internal auditor Sustainability, SMETA Certifications: ISO 9001, FSC, ISO 14001 & OHSAS 18001, 45001, 27001